.svg)
Sterilization Wraps

PRODUCT DESCRIPTION
PRIMED Sterilization Wraps are made of high-quality SMS (spunbond-meltblown-spunbond) polypropylene, combining strength and superior filtration capabilities to provide a barrier to protect medical devices from contaminants. PRIMED materials offer a smooth finishing pattern to reduce friction on skin and are highly drapeable, allowing for an easy aseptic presentation.
Available in a variety of sizes and types, there is a convenient option to meet various sterilization needs in alignment with standard hospital practices:
- PRIMED Single Layer Sterilization Wraps (S) are supplied to the customer as bulk packages of single sheets. Two sheets are then used to wrap a medical device or a collection of medical devices for sterilization.
- PRIMED Fused One Colour Sterilization Wraps (F1) are comprised of two sheets of PRIMED Single Layer Sterilization Wrap ultrasonically seamed on two sides. This allows for convenient wrapping with two sheets simultaneously.
- PRIMED Fused Two Colour Sterilization Wraps (F2) are made of two sheets in different colours ultrasonically seamed together.
Table 1: Dimensional Specifications of the Wraps
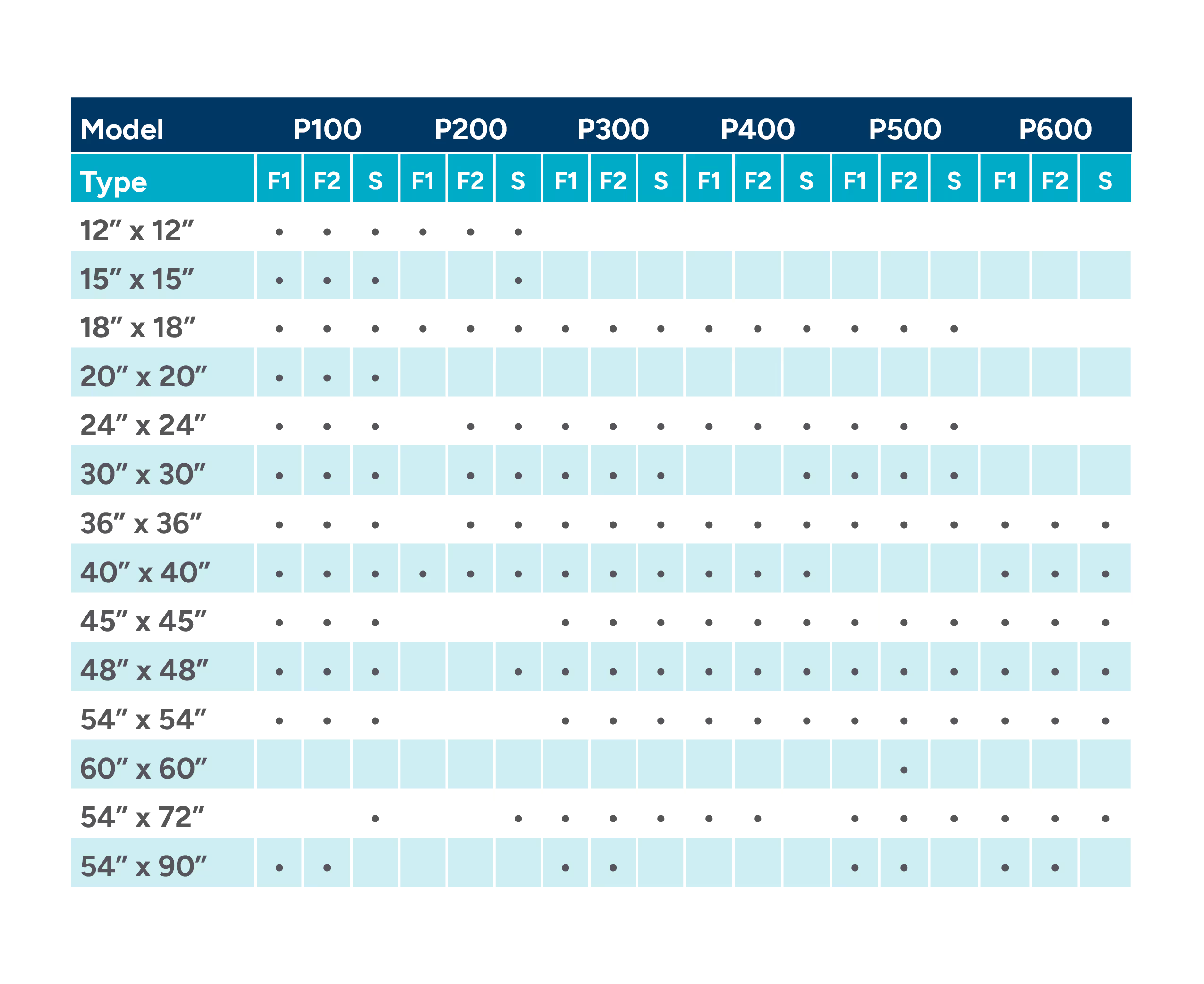
INSTRUCTIONS FOR USE
PRIMED Sterilization Wraps are intended to enclose medical devices to be sterilized by a health care provider using:
- Pre-vacuum steam at 270°F/132°C for 4 minutes and extended cycles up to 20 minutes. Validated for dry times of 20 minutes for P100 and P200, and for 30 minutes for P300 to P600.
- 100% ethylene oxide (EO) with a concentration of 725-735 mg/L at 131°F/55°C and 40%-80% relative humidity for 60 minutes. Validated for aeration times for EO sterilization of 8 hours at 55°C or 12 hours at 43.3°C.
- Lumen, Non Lumen and Flexible Cycles in the STERIS V-PRO Low Temperature Sterilization Systems.
- Advanced Sterilization Products STERRAD® Sterilization Systems:
- STERRAD® 100S [Long Cycle, Short Cycle]
- STERRAD® NX® [Standard Cycle, Advanced Cycle]
- STERRAD® 100NX® [Standard Cycle, Flex Cycle, EXPRESS Cycle, DUO Cycle]
- STERIZONE VP4 Low Temperature Sterilizer
The wrap is intended to allow sterilization of enclosed medical device(s) and to maintain sterility of enclosed device(s) until used.
GENERAL DIRECTIONS FOR USE
PRIMED Sterilization Wraps are designed to meet the requirements of the following standards:
- CAN/CSA Z314: Canadian medical device reprocessing
- ANSI/AAMI ST79: Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities
- ANSI/AAMI ST41: Ethylene Oxide Sterilization in Health Care Facilities
- AORN Standards, Recommended Practices, and Guidelines
- EN 868-2
- ISO 11607-1
It is recommended to follow the guidance in these standards when using PRIMED Sterilization Wraps, including but not limited to preparation, wrapping and sterilization chamber loading recommendations.
PRIOR TO USE
- Store wrap at room temperature and humidity for at least two hours before use.
- Inspect wrap for any irregularity, damage or extraneous particulate. Discard if any of these are detected.
- Ensure devices are adequately cleaned and dried before wrapping.
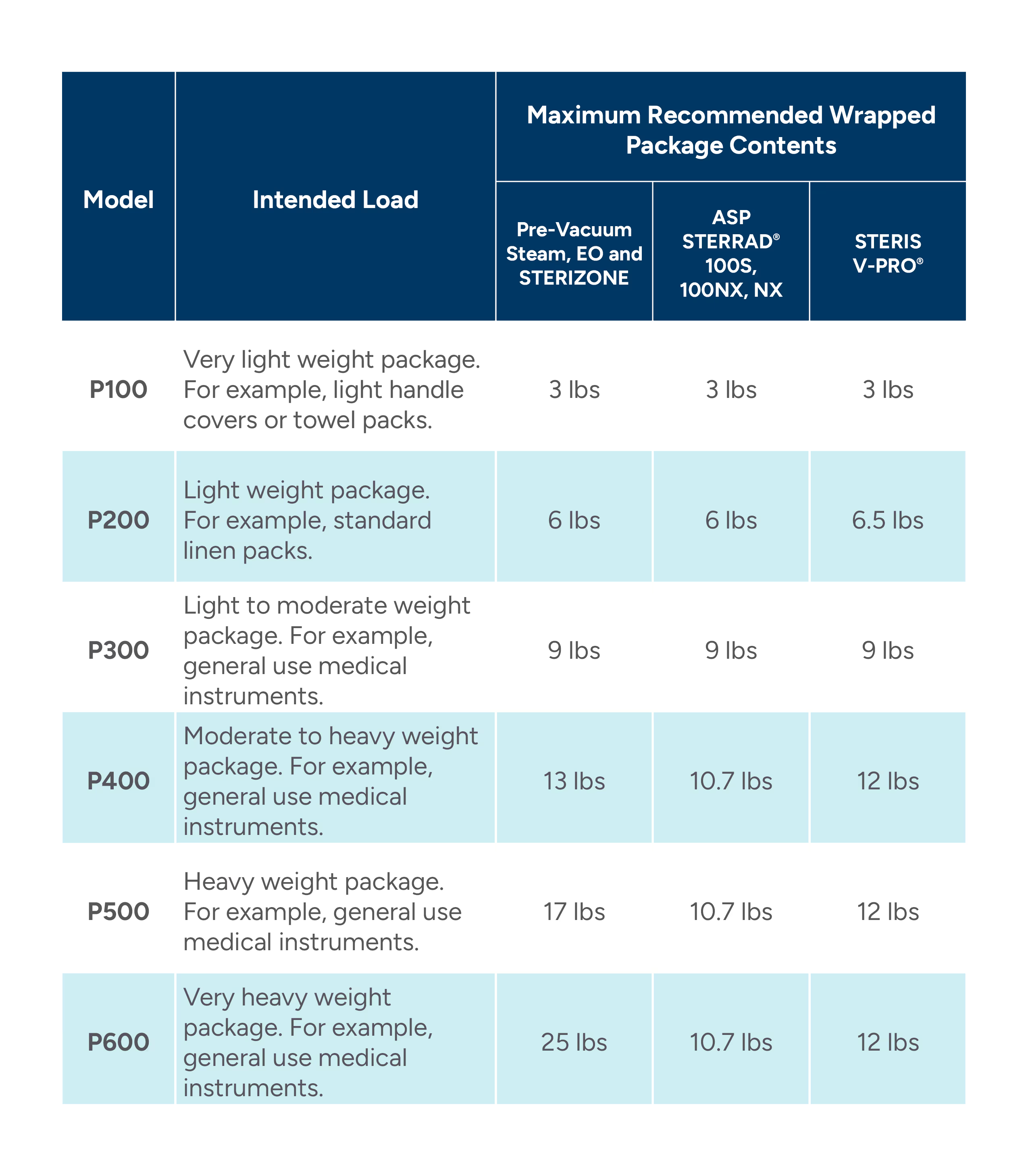
WRAP SELECTION RECOMMENDATIONS
The table below outlines the maximum recommended load for each material weight in different sterilization methods. Additional factors such as facility handling practices, storage conditions, wrapping methods, sealing methods, content shape or size, and sterilizer loading may influence the tolerable weight limits. Facilities should develop departmental instructions for use indicating appropriate wraps for each medical device and intended load.
Overall, it is highly recommended that the weight of the final wrapped contents does not exceed the maximum loads indicated below.
Table 2: Wrap Load Recommendations
WRAPPING TECHNIQUES
PRIMED Sterilization Wraps are compatible with common wrapping methods. It is recommended to refer to consensus standards such as CSA Z314 Section 15 or ANSI/AAMI ST79 Section 19 for wrapping instructions.
STERILIZATION PRACTICES
- PRIMED Sterilization Wraps are intended for use with the common healthcare sterilization cycle parameters. The Indications for Use outlines the parameters PRIMED Sterilization Wraps were validated with. It is recommended to consult with the sterilizer manufacturer for appropriate sterilizer loading configurations.
- If a sterilizer malfunctions or a cycle is aborted before completion, wrapped contents should be re-wrapped and re-sterilized.
- The validated drying times with pre-vacuum steam sterilization are 20 minutes for P100 and P200 and 30 minutes for P300 to P600.
- Disclaimer: The drying time may be affected by many factors such as device loading configuration, sterilization machine loading configuration, sterilizer performance, variations in cycle, temperature distribution, steam generation, altitude and ambient environmental conditions. See medical device manufacturer and sterilizer manufacturer MIFUs for guidance on dry times.
POST STERILIZATION RECOMMENDATIONS
- After sterilization, leave packages on the sterilizer cart until they are cooled to room temperature. It is recommended wrapped packages are not touched or moved until cooled.
- As wrapped items are removed from the sterilizer cart, carefully inspect each wrapped package for signs of damage. Any wrap with physical damage or signs of moisture should not be used.
- Special care and attention should be given when removing packages from shelves. It is recommended to use shelf liners for storing wrapped packages.
STERILITY MAINTENANCE
- Real time testing with PRIMED Sterilization Wraps supports maintenance of package integrity and sterility for 180 days after pre-vacuum steam, EO, STERIZONE, STERIS V-PRO and STERRAD sterilization. This shelf life is event related and users should follow established healthcare facility protocols.
OPENING
- Follow the health care facility’s policy for transport and handling of sterile packages.
- Before opening, examine the package for damage, wetness, or any sign of potential contamination. If these conditions are found, sterility could be compromised and the contents should not be used. Re-sterilize contents with a new wrap.
- Once opened, inspect the wrap again for any damage, wetness or potential contamination. Ensure sterilant penetration was effective by reviewing the internal sterilization indicator.
- Follow the healthcare facility’s practices for opening the packages aseptically.
DISPOSAL
- PRIMED Sterilization Wraps are single use products and are not intended for re-use. PRIMED Medical Products is not responsible for any resulting consequences from re-use of PRIMED Sterilization Wraps.
- Soiled wraps should be disposed of according to facilities’ internal and legal regulations protocols.
- Clean PRIMED Sterilization Wraps are eligible for recycling under the recycling classification code of 5 for polypropylene plastic. Soiled wraps are ineligible for recycling.
GENERAL STORAGE RECOMMENDATIONS
- Store unused wraps in a clean, dust free environment and protect from fluorescent or ultraviolet light.
- Use standard first in, first out (FIFO) stock rotation practices
- For post sterilization storage conditions refer to guidelines in standards such as CSA, ANSI/AAMI and AORN
WARNINGS
- Do not use wrap if there are signs of damage or external particulates found before use.
- Do not use in the incompatible sterilization methods of dry heat or radiation sterilization.
- Do not use the contents of a wrapped package if it is damaged or mishandled.
- Do not use in the presence of flammable anesthesia or materials. The wrap is anti-static and non-conductive.
PRECAUTIONS
- It is recommended to avoid the use of sharp knives when opening wrap packaging since knives may cut a wrap.
- Before wrapping, please consult medical device sterilization instructions to ensure they are compatible with and sterilizable by the sterilization modality and cycle listed above in the Indications for Use.
- Certain medical devices may require additional attention for their loading configuration prior to wrapping. See medical device manufacturer MIFUs for guidance on loading configurations.
- When sterilization takes place at an external facility, additional care and measures are recommended to protect and secure wrapped packages during transport. Follow the health care facility’s policy for transport and handling of sterile packages from an external source.
FAQs
Related Resources
Overhead Thumbloop Gown (Open Back)
Need information about best practices, shelf-life, donning and doffing? Find information on best practices for PRIMED’s medical & isolation gowns.

Overhead Thumbloop Gown (Full Back)
Need information about best practices, shelf-life, donning and doffing? Find information on best practices for PRIMED’s medical & isolation gowns.

Chemotherapy Drug Tested Gown
Need information about best practices, shelf-life, donning and doffing? Find information on best practices for PRIMED’s medical & isolation gowns.



